More than 200 million people around the world currently take statins aimed at preventing cardio-vascular disease; and the numbers are constantly rising as the criteria for being “at risk” are loosened. But what does the evidence say about the effectiveness of statins and about the balance between effectiveness and possible adverse side-effects? John Worrall – a long-time analyst of evidence in medicine – has recently had personal reason to reconsider these questions.
Statins, Strokes and Me – Part I: the intellectual phase
A good part of my later career was spent analysing the methodology of clinical trials. As part of which, statins and I have a drawn-out, uneasy, intellectual history. Large, double-blind randomised trials are generally thought of as the epitome of proper evidence-based medicine. However, as I pointed out in earlier work (e,g, this paper), application of some basic principles of the logic of theory and evidence quickly reveals significant methodological mistakes in the performance and interpretation of many such trials. This is true in particular of some famous large trials on treatments for, and prevention of, cardio-vascular disease (Heart Attacks and (Ischaemic) Strokes), including trials that are widely regarded as “establishing” the effectiveness of long-term statin use for the prevention of those diseases.
Examples of the methodological malpractices that have been involved in such trials are:
- The use of surrogate outcomes: What you are really interested in is the effect of some treatment on Cardio-Vascular (CV) mortality, or, still better, all-cause mortality. However, treating people for some number of years and waiting to see if they are still alive at the end of the period is costly and time-consuming. Much more efficient, then, to substitute, say, lowered blood cholesterol, as the outcome of interest – since changes in this can be readily and rapidly observed. However, you don’t want lowered cholesterol, you want not to have a fatal MI or stroke.
- The use of composite outcomes: again the outcome of real interest is the effect of some treatment on CV mortality, but some trials have quietly substituted for that outcome, a composite (more properly, disjunctive) outcome such as either fatal or non-fatal heart attack/stroke or being judged, at some time during the trial period, to need stents fitted to widen one or more arteries; a statistically significant difference in the treatment and placebo groups in the numbers of those experiencing the outcome leads to the “result” that the treatment is “effective”; but a little digging reveals that this difference is largely due to decreases in the treatment group in the numbers of non-fatal heart attacks and, more especially, of those being judged to need stenting rather than those suffering fatal CV events.
But by far and away, the most significant methodological malpractice is
- The use of relative rather than absolute risk reductions as the stated outcome of the trial – thus giving massively inflated impressions of the true size of the effect of the intervention.
Taking an extreme case: suppose a population of 1 million is being treated with statins and that, without treatment, 1 person in that population will experience a stroke or heart attack in, say, the next 5 years. (Of course, it is essential to the problem that we don’t know who that one person is – otherwise we could just treat him or her rather than the whole population.) Suppose furthermore that If everyone in the population is treated with statins for 5 years, no one will have a stroke/heart attack. The relative risk reduction: i.e., the percentage of those who would have experienced the negative outcome had they not been treated but who, with treatment, do not experience that outcome, is then 100%: all of those who would have had the stroke/heart attack are saved from it. Who would not want to take a treatment that is “100% effective” in preventing some dire condition that might otherwise afflict them? But of course “all of those who are saved” here amounts to one person: the relative risk reduction may be 100% but the absolute risk reduction is only 1 in a million. A randomly selected individual’s chance of benefitting from taking statins for 5 years is 1 in 1m. (Admittedly this assumes that the only benefit from taking statins is prevention of a stroke or heart attack. But, so far as I am aware, there is no telling evidence that there is any notable subsidiary benefit in this case – in contrast to, say, stopping smoking as a preventative measure for lung cancer, where there are any number of positive benefits of the intervention aside from making lung cancer less likely.)
Absolute risk can be re-expressed in a way that perhaps makes clearer the real meaning of the numbers involved: this is number needed to treat (NNT). In our extreme example, where the absolute risk reduction was 1 in a million, the NNT= 1,000,000 – meaning that you would have to treat 1 million people in order to achieve (on average) one positive outcome. In earlier papers, I recommended a different, though again logically equivalent, measure as likely to emphasise the true significance of the result still more vividly – namely, number treated ineffectively (NTI). Again, this is just a logical transformation of either absolute risk or NNT- in fact, NTI is always equal to NNT minus 1. In our example, NTI = 999,999: on average, you would need to treat 999,999 people ineffectively (at least in terms of stroke/heart attack prevention) in order to produce one positive outcome. A person’s being “treated ineffectively” means that they receive the treatment, and hence are exposed to possible adverse side-effects of that treatment but derive no benefit: since they would not have experienced the negative outcome (here stroke/heart attack) even if they had not been treated.
Or suppose to make the example slightly more realistic, that without statin treatment, 30 of our population of 1m will have a stroke/heart attack in the next 5 years, whereas if all are treated, that number will be reduced to 10. The relative risk reduction – stroke/heart attack is prevented for 20 out of 30 people – is a (seemingly) whopping 67%. But it is clearly not true that a random person taken from the population is 67% less likely to suffer a stroke/heart attack if they take a statin for 5 years. To make that inference would be to fail to take into account the “base rate” and hence to fall into the “base rate fallacy”. The real reduction in probability, the absolute risk reduction, is a miniscule 20 in a million. Or, in the perhaps more vivid terms of NNT: 50,000 people would on average have to be treated in order to prevent just one of them suffering a stroke/heart attack; hence, in order for just one person to have a CV event prevented, 49,999 people (NTI) would have been exposed to possible adverse side-effects of the statin without deriving any benefit from it (they were not going to have a stroke/heart attack in any event).
If you consult your doctor about whether you could benefit from taking a statin, then, depending on your supposed level of risk, you are likely to hear figures for risk reduction of 30, 40 or even 50% bandied about. Knowing that these are figures for relative risk, that the real absolute risk reduction was at best, and depending somewhat on your circumstances, in low single figures, and that statins, like all treatments, may produce notable adverse side-effects (of course these are crucial and more on them soon), I have long been a “statin sceptic”.
Statins, Strokes and Me – Part II: Now it’s Personal (High Risk/Low Risk)
It was, then, something of an irony when, in March this year, I suffered first a TIA (transient ischaemic attack or “mini-stroke”) followed within a couple of hours by a full-blown (ischaemic) stroke; and, a few days’ later, emerged from the National Neurological Hospital at Queen’s Square with great gratitude for the prompt and effective treatment that I had received, and a bag of pharmaceutical “goodies”, that I was supposed to take “life-long”. That takeaway lucky bag naturally contained a high-dose statin (Atorvastatin, 80mg): the issue of statins and CVD had become – very – personal.
When I expressed a guarded scepticism about statins to those taking care of me, I mostly received the standard reply – that statins had been “scientifically proved” to be effective in preventing strokes and also “proved” to be “remarkably safe”. (This is what the accepted guidelines tell them, citing the apparently impressive figures for relative risk reductions for benefits, though, tellingly, (alleged) absolute risk increases for adverse side-effects.) However I also occasionally got a more measured and more challenging response: “let’s grant you, for the sake of argument, that there are some grounds for scepticism about the ongoing drive to extend statin treatment to those at lower and lower risk, but when it comes to people at high risk – such as now yourself – the evidence is completely barn door: as someone who has already had a stroke you are definitely high-risk, and really ought to take a statin (life-long).” So, I took my statin (pro tem) and started my research again, concentrating on statins for those (deemed to be) at high risk.
Since most of the research has been done on heart attacks (or on a composite of either heart attack or stroke) rather than on strokes alone, it will help to explain here that heart attacks and (ischaemic) strokes are structurally similar afflictions. The etiology of both conditions involves the build-up of fatty atherolsclerotic plaques on the walls of an artery thus narrowing it. In the case of a “standard” heart attack, a plaque forms in a coronary artery, and eventually bursts leading to a blood clot forming on top of it – the clot blocking the artery and hence the blood supply to the heart. In a “standard” ischaemic stroke, an atherosclerotic plaque builds up in one of the carotid arteries in the neck, again the plaque breaks down and a blood clot forms, but this time, in contrast to a heart attack, the clot breaks away and travels into the brain, where it jams as the artery (naturally) narrows. The blood supply to some part of the brain is thus blocked. Because of the similarities between the two conditions, it is generally assumed in medicine that if statins are effective for either condition then they will be effective for the other.1 So, in this case in line with medical orthodoxy, I took it that trials on the effect of statins both on the incidence of strokes and on the incidence of heart attacks (or of course both) were relevant to my case. And in fact, given that heart attacks are considerably more common than strokes, trials on the former are understandably more common than trials on the latter.
The statin study that, sociologically speaking, seems to have carried the most weight concerning those at “high risk” was the “4S” study published in the mid-1990s . This involved 4444 Swedes who were assumed to be at very high risk of an heart attack since they had very high cholesterol levels and had already had a heart attack leading to an MI (myocardial infarction). The result, described by Suzanne Opril, then President of the AHA (American Heart Association), as “dynamite” that “would change medical practice forever” was an “almost half” reduction in the risk of a fatal coronary in the statin group (in fact 41%). But, as always, this was a relative risk reduction and the absolute risk reduction (for cardiac death) was an altogether less impressive 3.5% (and the ARR that you really want to know about – viz., that for all-cause mortality – a lot lower still).
A second trial that was also greatly influential and of even more direct relevance to me (the “elderly” were excluded from the 4S Study) is the PROSPER study performed a few years later. PROSPER looked at the effect of statins on 70-82 year olds with a history of risk factors for, or a history of, heart attacks. The result, which, according to the chief researcher James Shepherd, “extended to elderly individuals the benefits of statins already demonstrated for the middle-aged” was a 21% reduction (cardiac deaths). But, same old story, this is a relative risk reduction and Absolute risk reduction (cardiac death) = 0.9% and ARR (all cause) = 0.3%. (Interestingly, for women no effect was observed at all.)
An absolute risk reduction of 0.3%, translates into an NNT (number needed to treat in order to produce one positive outcome, remember) of 333 or, using the measure I have recommended, an NTI (= Number Treated Ineffectively) of 332. In other words, the PROSPER result implies that, for every “elderly” (male) patient who would have died without treatment with statins, you need, on average, to treat 332 such people who will derive no benefit (independently of treatment, they were not going to die of CVD in the relevant period anyway) but who may well suffer from any adverse side effects of the treatment.
A more recent study even found that, for people aged 65 or above, treatment with an LDL-lowering agent (which is what a statin is) actually increased all-cause mortality. (“Cholesterol” is a tricky concept – what doctors worry about and what statins lower is the level of LDL – low density lipoproteins [aka “bad cholesterol”].)2
Of course, no one should take the estimated effect from any individual study as gospel, but it does seem reasonably clear that, while the risk reduction for those deemed to be at high risk is approximately double that of those at low risk, it is still in absolute terms very small indeed. And, putting all the latest studies together (in so far as the pharmaceutical companies have granted access to the data), it looks like reasonable estimates, restricting ourselves to the really significant figures, absolute risk reductions (ARR)) are:
Primary prevention (i.e. prevention in people who have not already had a Heart Attack/Stroke):
- ARR (stroke): 0.4%
- ARR (MI): 0.9%
- ARR (all cause): 0
Secondary prevention (i.e. prevention in people who have already had a Heart Attack/Stroke):
- ARR (stroke): 0.8%
- ARR (MI): 2.6%
- ARR (all cause): 1.2%
So, although statins seem to be twice as effective for secondary care as for primary – twice almost nothing is almost nothing. (Moreover, the numbers are even closer to nothing for us “elderlies”.)
An Obvious Response: a second Stroke would be BAD, side-effects are rare and comparatively minor
There is, however, an obvious response to these figures: even a 1.2% reduction in my risk of a further stroke would be worth having IF the probability of adverse side-effects were next to zero. Moreover, a second stroke would, of course, be a very bad thing – so, if the adverse effects of the statins are comparatively minor, then the reduction in the expected (dis)utility of a second stroke, even if the reduction in the probability is very small, might outweigh the expected disutilty of a few aches and pains and muscular weaknesses. And this might be so even if the probability of those adverse effects being produced by taking the statin is reasonably high. And there is no doubt that there are many people who have been on statins for years without anything, or anything very much, by way of an adverse side-effect.
This was why I initially decided to take the prescribed statin albeit experimentally (also I was probably low on autonomy in the immediate aftermath of the stroke). But within a few days, I developed sharp pains in my heels and toes (which I had never had before) and was starting to notice some muscle weakening. I also had fairly extreme lethargy, though it’s obviously unclear whether that was attributable to the statin or just to having had a stroke in the first place.
The reaction from my doctors was “okay, but there are lots of different statins (and different dose levels and different LDL-lowering agents), let’s see if we can find one that you ‘tolerate’ better”. I gave this, a priori entirely sensible, suggestion a lot of thought.
The problem is that there is some quite telling evidence that, not only are the immediate side-effects remarkably common and certainly unpleasant, but also long-term statin use can lead to some very nasty side-effects indeed: Parkinson’s, MS and Motor Neurone disease, arguably amongst them. The line that medical orthodoxy takes is that the evidence for adverse effects in general is all “merely anecdotal” (or possibly a set of instances of the nocebo effect – people suffer from adverse effects only because” anti-statin propaganda” had led them to expect to suffer those effects); and that the “real” evidence (from blinded clinical trials) is that there is no significant difference in adverse events between those taking statins and those taking placebo: that in fact, despite internet-fuelled scare stories, statins are generally remarkably “well-tolerated”.
Adverse Events: Perhaps Not So Rare, Not So Minor
There are (at least) three things to say about this. First, while there are clinical trials that have identified adverse side-effects, particularly of extreme fatigue and aggression, the majority of clinical trials have, admittedly, not “identified” significant side-effects of statins. However, it should be noted that clinical trials in general are ill-equipped to pick up side-effects of treatments. First of all, “you have to ask the question to get an answer” and few trials are geared toward looking for side-effects. Moreover, so far as immediate or short-term adverse effects are concerned, the big trials invariably involve a “run-in period’”– those participants who exhibit side-effects to the trial treatment during that period being excluded from the “trial proper”. Also, the more serious adverse effects are likely to show up only with long-term use, so any short-term trial will not pick them up, and even trials that last for up to 5 years may not be long enough for the side-effects to develop.
This is a genuine worry, not an idle “what if?”, as shown by the case of Cerivastatin. Just as you can now read any number of comforting reassurances from medical experts that the current crop of statins are essentially free of significant adverse side-effects, you could read back in 2000:
“Cerivastatin is generally well tolerated and adverse events have usually been mild and transient. The overall incidence and nature of adverse effects with cerivastatin in clinical trials was similar to that of placebo.”
Cerivastatin became very widely prescribed (the dose level is much lower than for other statins); but very shortly afterwards, in 2001, it was withdrawn because of a large number of deaths associated with use of the drug from Rhabdomyolysis – a catastrophic breakdown of muscle-tissue, leading to kidney failure. Cerivastatin had been “validated” in clinical trials which had failed to identify any adverse side-effects.
So the fact that significant adverse effects have not shown up in most (though not all) clinical trials is by no means fatal to the claim that statin use causes significant adverse effects. It is, however, true that most of the (alleged) evidence for that claim comes, not from clinical trials, but from reports by patients. And these tend to be dismissed by hard-line supporters of Evidence-Based Medicine as “mere anecdotes”. But the second point to make here is that, despite this negative attitude, there is, of course, nothing automatically non-telling about “anecdotes” (aka what the patient tells the doctor about his or her experience) as such. (If “anecdotes” could form no serious part of the evidential picture in medicine it would be strange that doctors spend so long listening to patients relating their experiences.) The problem here is the basic one in evidence-assessment – alternative explanations: before taking it that an anecdote, or indeed any piece of alleged evidence, is real evidence for some causal claim, one should always ask “what else might it be?”.
There is, for example, any amount of documented anecdotal “evidence” that statins cause loss of libido: but, of course, people’s libido tends to reduce as they get older or if they experience a serious illness. By making the inference: you took statins and lost your libido, therefore, you lost your libido because you took statins, you commit the all-too-prevalent post hoc, ergo propter hoc fallacy (“after the event, therefore because of the event”). An evidential extra is clearly needed – namely, some reason to think that you would not have lost your libido if you had not taken the statin. But that is why it is a crucial fact about the “anecdotal” evidence concerning side-effects of statins that it consists, not just of considerable numbers of people reporting (alleged) adverse consequences of taking them, but also, almost invariably, of reports that their symptoms were reversed once they stopped taking their statins.
Thirdly, and for me most tellingly, there are very sound – evidence-based – biochemical reasons why you would expect precisely the adverse effects recounted in the “anecdotes” if you significantly reduce the body’s cholesterol (again really LDL, low density lipoprotein) production.3 To take one, example that I already touched on: there are (very) widespread reports of loss of libido amongst those taking regular statins (though loss of libido is not officially listed as a possible adverse side-effect). But we know from biochemistry that LDL cholesterol is required to synthesise both testosterone and the female sex hormone oestrogen : that is, we know that the production of testosterone and oestrogen lies downstream from synthesis of LDL cholesterol in the (unobstructed) biochemical cascade originating in the liver. So, what would you expect to happen to a man or woman’s ’s libido if you significantly reduce his or her body’s production of LDL cholesterol?
Moreover, that biochemical cascade produces LDL at its 37th stage, and statins block the process very early, in fact at stage 2: so they also block the production of lots of other important chemicals besides those that are directly downstream from LDL.
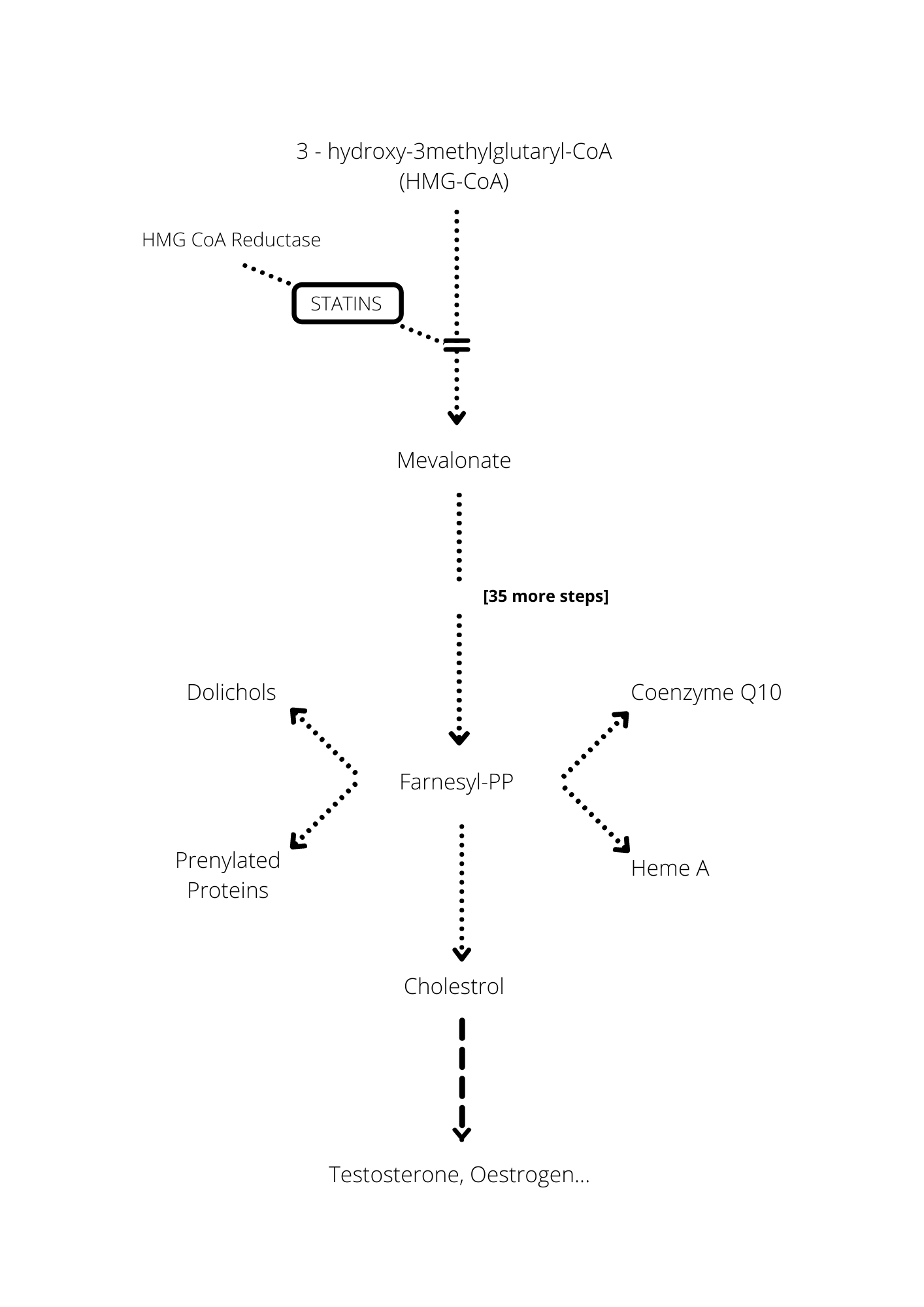
Cholesterol synthesis: statins block the production of mevalonate – at the second stage of the 37-stage process involved in the synthesis of cholesterol; the diagram indicates some of the critical substances (notably Co-enzyme Q10) other than cholesterol whose production is thereby also blocked; the synthesis of testosterone, oestrogen and other important substances occurs naturally downstream from the synthesis of cholesterol and is therefore also blocked by the statin.
Not the least of these important chemicals is CoQ10 (aka “Q10”), primarily used by the mitochondria and hence crucial for the body’s energy production. Loss of energy/lethargy/muscle weakness and pain is a massively frequently reported “consequence” of taking statins; recent Danish research showed that “patients examined who were being treated with statins had low levels of the key protein Q10. Q10 depletion and ensuing lower energy-production in the muscles could be the biological cause of the muscle pain that is a problem for many patients.” Indeed it could.
Finale: Worrall Takes a Decision
So, Dear Reader, I took the decision to stop my statin permanently – to widespread medical incredulity.4 I do not, of course, claim that this is the uniquely rational decision to make on the basis of the available evidence. In order to substantiate that claim (via an expected utility calculation) you would need access to probabilities – such as the probability of developing Parkinson’s or Motor Neurone Disease if you take a statin long term – for which there are currently no reasonable evidence-based estimates. Still more, you would need some sort of reasonable measures of the relative (dis)utilities of horrible conditions such as Stroke and Motor Neurone Disease.
What I do think is established by any dispassionate survey of the current evidence is that the black and white view currently accepted in, and (strongly) advocated by, medicine – namely, that taking a statin is the uniquely rational decision for someone like me – is untenable. If we put together the evidence about the extent of the reduction in risk of a further stroke (or heart attack) brought about by statins and the evidence concerning statins’ adverse side-effects, then it is surely not unreasonable to decide that the costs of taking a statin are, not certain, but likely, to outweigh its benefits.
By John Worrall
Having been in LSE’s Philosophy Department – student and teacher – for over 50 years, John Worrall is now Emeritus Professor. His main intellectual interests continue to be issues arising from theory-change in science (especially “structural realism”) and, as this post reflects, evidence in medicine.
Further reading
- Malcom Kendrick’s books The Great Cholesterol Con and the more recent A Statin Nation argue the case against statins vigorously and in detail.
- The most clear-sighted general introduction to the analysis of risk in medicine is provided by Gerd Gigerenzer – I particularly like his earlier work Reckoning with Risk.
- The attitude toward statins is part of a more general tendency in medicine to overtreat – in particular to overtreat “risk”. James Le Fanu’s Too Many Pills should, in my view, be compulsory reading in Medical Schools in the attempt to put a brake on this tendency.
Acknowledgements
Grateful thanks to Alex Voorhoeve and Roman Frigg for helpful comments on a previous draft.
Notes
1 — Although as a matter of fact, if the data for the two conditions are considered separately, then they are (even) less impressive in the case of strokes, than they are in the case of MIs.
2 — For further details consult the books by Malcom Kendrick listed in the further reading.
3 — The most egregious mistake made by the founding fathers of Evidence Based Medicine was not the (arguable) overplaying of the significance of randomising (which was the chief focus of criticism), but rather the (arguable) underplaying of the significance of “patho-physiologic rationale”. Of course, the ultimate question about some treatment is whether it works in vivo and works without producing significant adverse side-effects. Moreover, science is of course fallible and considerations of the physiology/biochemistry of the effect of some treatment on some condition can occasionally turn out to be misleading.. (A very small number of such cases was what prompted the scepticism from David Sackett and other founding fathers of Evidence Based Medicine about the evidential impact of “pathophysiologic rationale”.) But clearly the evidence for the effectiveness of a treatment (or, as here, the involvement of a treatment in some adverse event) is much stronger if it consists not just of trial or observational results, but also of a background evidence-based physiological account of the causal connection between treatment and outcome (whether therapeutic or adverse).
4 — Of course, I say “permanent” here only to contrast this decision with my initial one to take the statin, which was explicitly temporary in my mind, pending a re-review of the available evidence. For the rational person all substantive views and therefore substantive decisions are defeasible by further, currently unenvisaged evidence. No view (outside of mathematics or logic) should ever be taken permanently – especially by a philosopher. As John Maynard Keynes said (or did he?) in response to someone who complained about his changing his mind, “When the facts change, I change my mind. And you, Sir?” (Some commentators claim that Keynes did not make this famous remark, and even if he did it is rather sloppily expressed – the facts do not change, the correct, if somewhat clumsier, thing to say is “When the evidence changes (that is, new currently unenvisaged evidence emerges), I shall need to consider whether I should change my mind.”








































































































hi! your article is very informative and has good knowledge. good work….
hi! your article is very informative and has good knowledge. I am glad to see a wonderful piece of article….